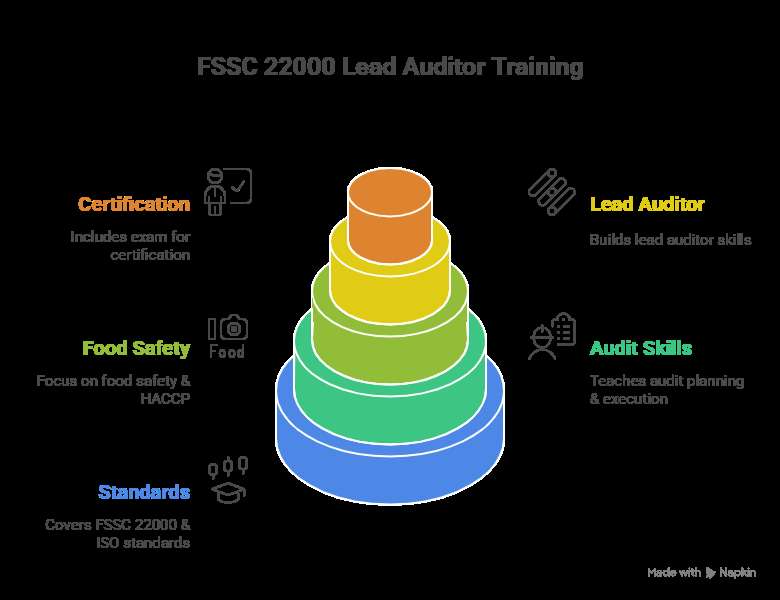
ISO certification is a formal recognition that an organization’s management systems, products, or services meet the standards set by the International Organization for Standardization (ISO). It helps businesses improve quality, efficiency, safety, and environmental impact, enhancing customer trust and opening doors to new markets.
https://iasiso-asia.com/VN/iso....-certification-in-vi